Join our team!
History
Start-up.
Orpheus LIS
Development of laboratory system.
Radius
Development of information system for radiological departments.
Amadeus
First installation of the Amadeus transfusion system.
Orpheus LIS
Laboratory information system put into operation.
Pathology
Development of an information system for pathology.
Transformation of Steiner, s.r.o.
Development of an information system for prometheus haematopoietic cell donor registries.
The Czech Registry of Haematopoietic Cell Donors in IKEM became the first registry in Central and Eastern Europe connected to EMDIS using the Prometheus system.
Cryus
Development of an information system for tissue banks and laboratories of hematopoietic cell processing
Development of the Orpheus HLA system for immunogenetic laboratories and transplant centers.
Quality Management System (QMS) certification according to ISO 9001 and Information Security Management System (ISMS) certification process according to ISO 27001.
ISBT 128 (Information Standard for Blood and Transplant) certification.
Perseus
Development of a system for workflow management of pathological laboratories.
Steiner, s.r.o. expands cooperation with ICZ
The company has customers in more than 40 countries around the world.
We have released a new system of self-registration of donors for the Finnish registry.
We became members of ČISOK
We have expanded our cooperation with EladHealth and become members of the Czech-Israeli Joint Chamber of Commerce.
Corporate Governance

Ing. Ariel Steiner, RNDr.
Company Director

Mgr. Ing. David Steiner, MBA, Ph.D.
Managing Director

Zuzana Holubova
Provozní manažerka & Manažerka kvality a bezpečnosti

Bc. Vladislav Malovičko
Project Manager

BS Albert Mustafin
Product Manager

Mgr. Ing. Martin Novák
Product Manager

Ing. Miloslav Kavka, MBA
Project Manager
Certificates
Certification
ISO 9001 and ISO 27001 certification
Steiner, s.r.o. has long been striving to improve the quality of its products and services. In 2011, we took a significant step in this direction – we successfully completed the certification of the quality management system (QMS) according to ISO 9001 and at the same time the certification of the information security management system (ISMS) according to ISO 27001. This is a guarantee for our Czech and foreign customers that we meet the demanding requirements of these standards and adhere to international standards in the development of information systems, the provision of services and the management of the company.
ISBT 128 Certificate
ICCBBA is an international non-governmental entity in official relations with the World Health Organization (WHO) that administers, develops and grants ISBT 128 licenses; an international information standard on the terminology, coding and labelling of medicinal products of human origin. ICCBBA manages the assignment of globally unique identifiers to licensed facilities and manages the ISBT 128 standard, international databases for device identification numbers and product description codes, supporting documentation, and educational materials.

European Union
Projects supported by EU funds

Steiner, s.r.o. implements the project "Global Search System of Haematopoietic Cell Donors Spol. Steiner", the aim of which is to create an international database that will connect several dozen national registries and enable simple, fast and reliable search for unrelated donors using a new Internet application. Financial support from the European Union (OPPIK) is provided for this project.
We also implemented the project "Development of Steiner Development Capacities" within the ICT and Strategic Services Program, which is part of the Operational Programme Enterprise and Innovation.
Steiner, s.r.o. implements the project "Development of the market for transfusion products", which aims to acquire new knowledge and procedures in the areas of transfusiology, automation and biomedicine. The new findings will be applied during multidisciplinary industrial research and experimental development of the Transfusion Products Marketplace software, which will enable the participating blood service facilities and healthcare facilities to optimize mutual sales, exchanges and purchases of transfusion products.

TAČR
Project with the support of the Technology Agency of the Czech Republic
The TRIMMUS (Transplant Immunology Decision Support System) project fulfils the objectives of the TAČR KAPPA program by expanding the cooperation between the Czech company Steiner, s.r.o. and the Department of Immunology of the University Hospital in Oslo, Norway.
The aim of the project is to develop a software solution completely covering the workflows of the Transplantation Immunological Laboratory of the University Hospital in Oslo so that it is possible to abandon paper methods of communication during routine manual procedures, increase patient safety while providing the best possible care, meet accreditation requirements, improve the flow of information and increase data security, and last but not least, speed up workflows and improve decision-making.
- Project number: TO01000057
- Project holder: Steiner, s.r.o
- Project partner: Oslo University Hospital
- Contact person: Mgr. Ing. David Steiner, MBA, Ph.D.
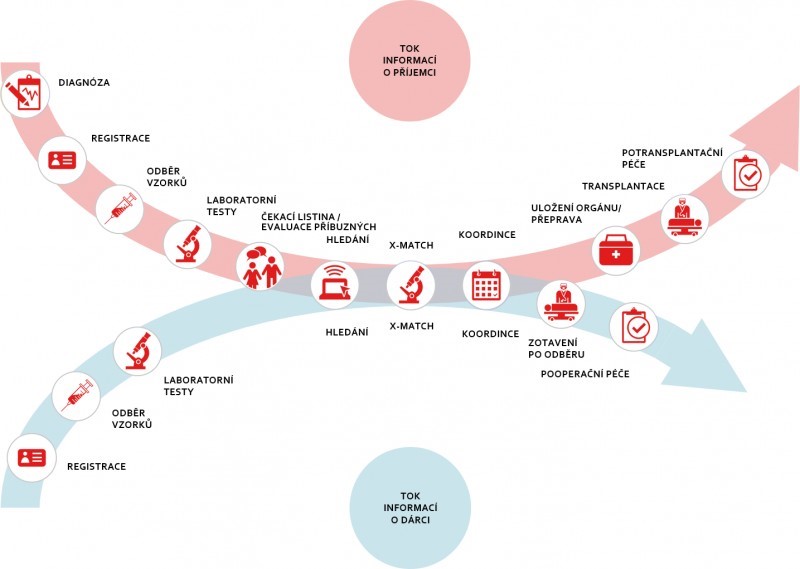
Transplant immunology is a medical science ensuring optimal donor-recipient matching before allogeneic transplantation of cells, tissues and solid organs. Allogeneic transplantation is a complex process involving transplant immunology, donor availability, appropriate criteria, coordination, and specialized medical staff. Oslo University Hospital requires a laboratory information system for transplant immunology, integrated with decision support systems, workflow mapping and post-transplant control registries.
Our team combines the IT skills and HLA experience of Steiner, s.r.o. staff with the laboratory and clinical knowledge of the medical staff of the University Hospital in Oslo. Since all transplant immunology, organ transplantation and HLA matching for stem cell transplantation in Norway take place in one center, we have a unique opportunity to develop and test the benefits of the new Transplant Immunology Decision Support System (TRIMMUS). This includes all the steps from registering samples in the Transplant Immunology Laboratory through decision support tools to connecting available donors with recipients to evaluating long-term results after allogeneic transplantation. TRIMMUS replaces manual data transfer, reducing the risk of human error. TRIMMUS will also present all relevant data and run algorithms to facilitate fast and appropriate advice and decision-making, thereby providing the best possible care.
Steiner supplies the software technology, and the University Hospital of Oslo provides medical expertise to develop algorithms and evaluate the performance of each algorithm and the entire solution. The outputs of the post-transplant conclusions will be used to improve pre-transplant search algorithms.
Thanks
The Transplant Immunology Decision Support System (TRIMMUS) received €1.2 million in support from Iceland, Liechtenstein and Norway through EEA grants and the Technology Agency of the Czech Republic under the KAPPA programme.
News:
12.11.2024
Kappa program
Závěrečná konference
Dne 10. prosince se zúčastníme závěrečné konference programu KAPPA (program aplikovaného výzkumu realizovaný Technologickou agenturou ČR v rámci Grantů EHP a Norska), která bude sloužit jako rozloučení a zároveň symbolické zhodnocení výsledků programu, prezentace financovaných projektů a příležitost pro sdílení získaných zkušeností.
Bude také zaměřena na zkoumání budoucích vyhlídek a příležitostí, které se v rámci programu otevírají.
30.4.2024
S potěšením oznamujeme úspěšné dokončení projektu Transplant Immunology Decision Support System (TRIMMUS), softwarového řešení určeného ke zlepšení pracovních postupů a rozhodovacích procesů na odděleních transplantační imunologie. Tento projekt, vedl ke zlepšení pracovních postupů při transplantaci orgánů a tkání.
Systém TRIMMUS je komplexní platforma navržená tak, aby nahradila manuální, papírové postupy plně digitálním a efektivním pracovním postupem. Zahrnuje pokročilé bioinformatické algoritmy, jako jsou PRA, CRF a EPF, pro optimalizaci identifikace vhodných dárců, zvýšení bezpečnosti pacientů a zlepšení klinických výsledků.
18. – 21. 6. 2023
Projekt TRIMMUS (Transplant Immunology Decision Support System) byl prezentován v rámci posterové sekce na nedávném setkání Světové asociace dárců dřeně (WMDA). Na této prestižní akci se sešly světové špičky v oblasti transplantační imunologie a správy registru dárců a poskytla vynikající platformu pro prezentaci inovací projektu TRIMMUS.
Poster zdůraznil klíčové vlastnosti systému TRIMMUS, včetně jeho automatizovaných algoritmů pro vyhledávání dárců, pokročilých nástrojů pro typizaci HLA a jeho schopnosti optimalizovat rozhodovací proces před a po transplantaci. Konference nám poskytla příležitost diskutovat s kolegy z transplantační komunity o budoucí spolupráci a možnostech integrace systému TRIMMUS do jejich laboratoří.
22.02.2022
To date, we have improved the conditions for testing the functionality of the future application. On Steiner's side, a new virtual server has been set up with significantly higher performance than the existing one, so we can perform a larger number of automated tests every day. At Oslo University Hospital, current data from the production server has been copied into the development environment, and on them the functionality of the application and the new algorithms will be verified by bioengineers and doctors involved in the project. At the same time, on this occasion we will verify the correctness of the data migration to the current database schema
07.10.2021
After ten months of intensive work since the start of the project, the implementation phase of the first tasks of the new TRIMMUS (Transplant Immunology Decision Support System) product has been completed. In the field of bioinformatics research and development, an algorithm for searching for platelet donors and algorithms for determining PRA, CRF and EPF have been completed. In the field of software research and development, several hundred changes were made to the user interface, and more than 2000 automated tests were created to machine verify the correct functionality of the entire application.

